Our Technology
A leader in antibody drugs in China with capabilities in research and development, manufacturing and commercialization.

Our Technology
A leader in antibody drugs in China with capabilities in research and development, manufacturing and commercialization.
Professional Advantages and Excellent Experience
-

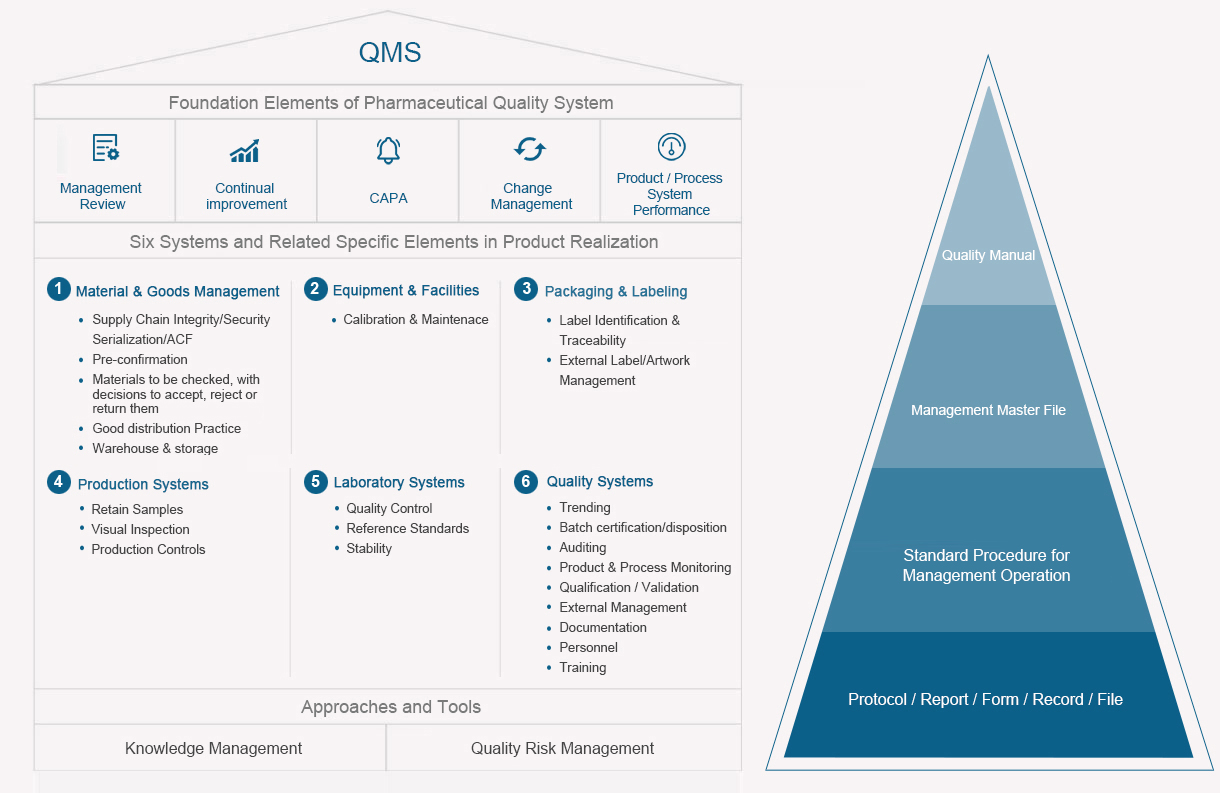
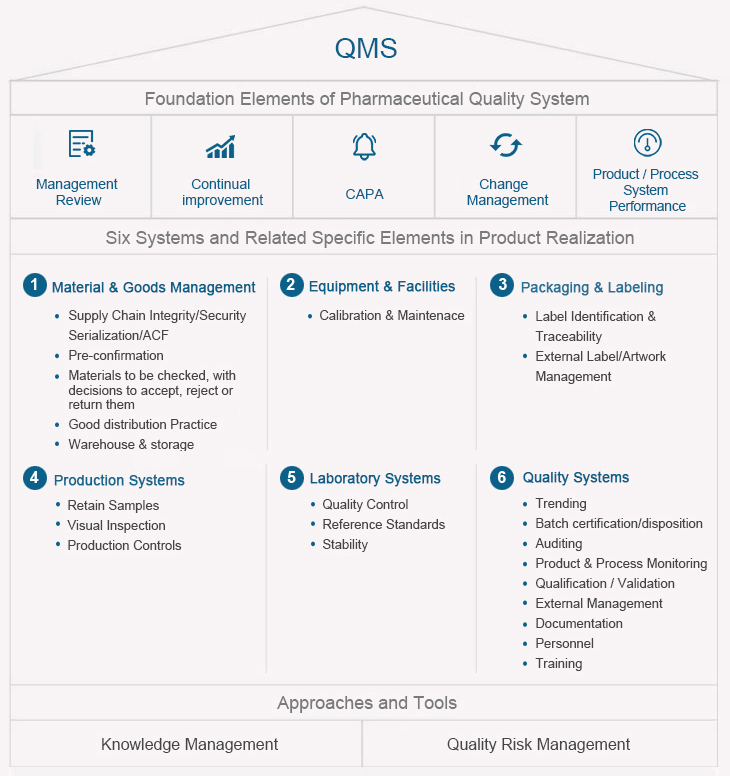
Taking product quality as the lifeline, Sunshine Guojian has formulated the quality policy of 「Led by science and technology, winning by quality, customer first, pursuing excellence.」 The company adopts advanced science and technology, strictly implements relevant drug management regulations and standards, and consults relevant technical guidelines, so as to ensure the effectiveness, safety and quality stability of its drugs, allowing patients to benefit from more effective, affordable, high-quality, safe and reliable drugs.
-

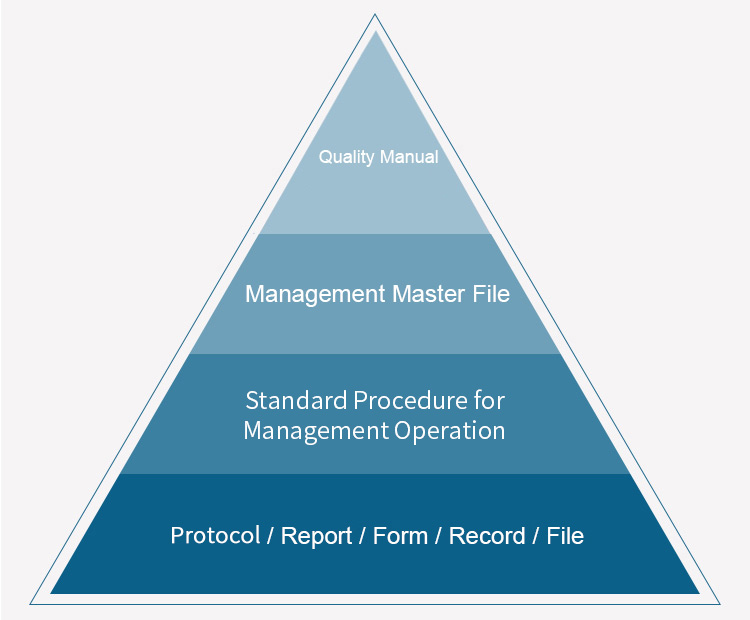
The company has formulated complete production and quality management rules and regulations according to the Chinese GMP standards, as well as guidelines of the U.S. FDA and European Medicines Agency, covering document system, employee training, verification and validation, risk management, change control, annual product review, corrective and preventive measures, materials management, production operation, production management, quality control, product storage, shipment and self-tests. It has also established the quality standards, control standards, analytical methods, process procedures and other technical regulations for raw and auxiliary materials, packaging materials, intermediate products and finished products. With a series of management standards and operation procedures, the issuer has realized the standardization, routinization and institutionalization of all production processes by conforming to the high-standard GMP management requirements, thereby guaranteeing the smooth progress of production.
Quality Management
Seasoned and Stable Quality Management Systems
Technology Capabilities:
Following ICH Q10 and FDA's ICH Q9 to design, enhance and maintain a robust and systematic QMS;
Knowledge management and quality risk management approach to drive continuous improvement and support;
Verified through inspections by NMPA and other national health agencies from Europe, North/South America, and etc.
Quality Control

Establishing Specification for Early Stage Development
Quantity and Potency
Strength (UV280, HPLC)
Protein purity (SEC-HPLC, RP-HPLC, CE-SDS)
Potency (Affinity, MOA related Cell-based assay , CDC, ADCC, Fc Receptor Function)
Establishing Specification for Clinical Use
Physiochemical
Appearance
General test: pH, osmolality, key excipients, particle
Identity : charge profile (CZE, IEC, cIEF) , peptide mapping, pI (IEF, cIEF)
Establishing Specification for Market Application
Structure
Intact mass (SDS-PAGE, CE-SDS, SEC-HPLC, DLS, LC-MS)
Peptide mapping and PTM (LC-MS/MS)
Glyco-profiling (HILIC-HPLC, CE-LIF, Chip-CE, LC-MS/MS)
Disulfide and free cysteins (LC-MS/MS, Ellman’s Assay)
N-terminal and C-terminal (Sequencing of amino acids by Edman degradation)
Post-translational modification (LC-MS/MS)
Higher structure (CD,DSC, DLS , fluorescence)
Routine Use and Maintenance of Approved Specification
Impurities/Contaminants
Aggregate/product variants (SEC-HPLC, IEC, cIEF, HIC, RPLC)
HCP (ELISA)
Protein A (ELISA)
DNA (Q-PCR)
Endotoxin & bioburden
Sterility

Technology Capabilities:
Complete analytical and testing capabilities throughout the product life-cycle;
Analytical method development, transfer and validation ability complying to ICH/USP/EP/CP Guideline;
Strict quality control system covering IPC, Lot release and stability program;
Thorough comparability and quality research ability.
Our Technology
A leader in antibody drugs in China with capabilities in research and development, manufacturing and commercialization.

Our Technology
A leader in antibody drugs in China with capabilities in research and development, manufacturing and commercialization.
Professional Advantages and Excellent Experience
-

Taking product quality as the lifeline, Sunshine Guojian has formulated the quality policy of 「Led by science and technology, winning by quality, customer first, pursuing excellence.」 The company adopts advanced science and technology, strictly implements relevant drug management regulations and standards, and consults relevant technical guidelines, so as to ensure the effectiveness, safety and quality stability of its drugs, allowing patients to benefit from more effective, affordable, high-quality, safe and reliable drugs.
-

The company has formulated complete production and quality management rules and regulations according to the Chinese GMP standards, as well as guidelines of the U.S. FDA and European Medicines Agency, covering document system, employee training, verification and validation, risk management, change control, annual product review, corrective and preventive measures, materials management, production operation, production management, quality control, product storage, shipment and self-tests. It has also established the quality standards, control standards, analytical methods, process procedures and other technical regulations for raw and auxiliary materials, packaging materials, intermediate products and finished products. With a series of management standards and operation procedures, the issuer has realized the standardization, routinization and institutionalization of all production processes by conforming to the high-standard GMP management requirements, thereby guaranteeing the smooth progress of production.
Quality Management
Seasoned and Stable Quality Management Systems



Technology Capabilities:
Following ICH Q10 and FDA's ICH Q9 to design, enhance and maintain a robust and systematic QMS;
Knowledge management and quality risk management approach to drive continuous improvement and support;
Verified through inspections by NMPA and other national health agencies from Europe, North/South America, and etc.
Quality Control

Establishing Specification for Early Stage Development
Quantity and Potency
Strength (UV280, HPLC)
Protein purity (SEC-HPLC, RP-HPLC, CE-SDS)
Potency (Affinity, MOA related Cell-based assay , CDC, ADCC, Fc Receptor Function)
Establishing Specification for Clinical Use
Physiochemical
Appearance
General test: pH, osmolality, key excipients, particle
Identity : charge profile (CZE, IEC, cIEF) , peptide mapping, pI (IEF, cIEF)
Establishing Specification for Market Application
Structure
Intact mass (SDS-PAGE, CE-SDS, SEC-HPLC, DLS, LC-MS)
Peptide mapping and PTM (LC-MS/MS)
Glyco-profiling (HILIC-HPLC, CE-LIF, Chip-CE, LC-MS/MS)
Disulfide and free cysteins (LC-MS/MS, Ellman’s Assay)
N-terminal and C-terminal (Sequencing of amino acids by Edman degradation)
Post-translational modification (LC-MS/MS)
Higher structure (CD,DSC, DLS , fluorescence)
Routine Use and Maintenance of Approved Specification
Impurities/Contaminants
Aggregate/product variants (SEC-HPLC, IEC, cIEF, HIC, RPLC)
HCP (ELISA)
Protein A (ELISA)
DNA (Q-PCR)
Endotoxin & bioburden
Sterility

Technology Capabilities:
Complete analytical and testing capabilities throughout the product life-cycle;
Analytical method development, transfer and validation ability complying to ICH/USP/EP/CP Guideline;
Strict quality control system covering IPC, Lot release and stability program;
Thorough comparability and quality research ability.




